Rare Disease Day 2020
The last day of February is International Rare Disease Day – originally celebrated on Leap Day (February 29). So this is one of the truly rare Rare Disease days that only comes around once every four years. An excellent day to begin this blog about CADASIL.
I’m Nahum Smith, from the laboratory of Lea Starita and representing the Brotman Baty Institute for Precision Medicine (BBI). In this blog, I’ll be explaining our research on CADASIL: the disease, the gene, and the techniques we use at the lab bench and at the computer to understand them both.
Doing the Science
Since April of 2019, I’ve been developing a test to predict for pathogenic variants in Notch3, the predominant gene associated with CADASIL. This blog will follow my progress with the project, as well as delve into the complexities of the disease and others like it.
Let’s begin today with a breakdown of what CADASIL stands for, and why this protein (Notch3) is so important to it.
CADASIL stands for Cerebral Autosomal Dominant Arteriopathy with Sub-cortical Infarcts and Leukoencephalopathy. It’s a mouthful, so each of these words are worth addressing so we can progress together through understanding the biology of this disease.
Cerebral just means that the disease is of the brain.
Notch3 is the only gene with a prominent correlation to the disease. Notch3 is coded for on chromosome 19, one of the autosomal, or non-sex-linked chromosomes. We humans have two copies of each of our 22 autosomal chromosomes and only one of the sex-defining X and Y, so it’s not unusual at all for CADASIL to be an autosomal disease.
Fun fact: The 22 autosomal chromosomes are named in order of relative size (with Chr01 the largest), so Chr19 is relatively small – but it still contains over 1700 genes within over 60 million DNA base pairs. Notch3 is just one of those genes. Notch3 is also over 7,000 bases long, which is quite big for a single gene!
The dominant aspect is, however, quite defining. Many genetic disorders are recessive, meaning that if one of the two copies of a gene is present and functioning, that single copy will be sufficient to produce enough protein for healthy cell-function. This is common in most cancers, where disease progression occurs only after an accumulation of mutations in related genes. This is unfortunately not the case for CADASIL, where pathogenic mutations “overtake” normal functionality, meaning if you inherit the gene from one parent you will get the disease. The burden this has on families affected by the disease is substantial.
The fact that its autosomal-dominant puts CADASIL in a rare and unpleasant category of genetic disorders.
Arteriopathy is, simply put, a disease of the arteries. Arteries deliver oxygen-rich blood to the body’s organs, and the brain (remember – cerebral) is of the greediest of organs for oxygen delivery. While CADASIL is a neurodegenerative disease like Parkinson’s or Alzheimer’s, these other diseases primarily affect neurons, which are what most of us think of when we imagine the cells in our brains. CADASIL is different, though, in that primarily affects the cells – the blood vessels – around the neurons. Its effect is indirect – which makes measuring disease progression more difficult.
An infarct is a small area of dead tissue resulting from a failure of blood supply, so you could say that the arteriopathy causes infarcts. A sub-cortical infarct is one that would be “below the cortex”; the cortex is the outer, upper layer of the brain. A subcortical infarct is deep within the center of the brain. A more common term to describe this is a stroke.
This bring us to leukoencephalopathy – a disorder of white brain matter. White matter is found in the deeper tissues of the brain. So if you looked at an exposed brain, you wouldn’t see much of it. White matter is composed of a mass of connections between the disparate parts of the brain and gets its color from the fatty myelin sheaths that coat the neurons.
Put these all together and you’ve got a dominantly inherited disease that affects blood vessels deep within the center of the brain. That’s a lot to take in. Not only is CADASIL difficult to describe, it’s difficult to pinpoint how it begins and progresses.
Notch3’s Role in CADASIL
There is a known culprit, however, and that’s the protein named Notch3. The majority of individuals diagnosed with CADASIL have uncommon variants within the gene. In a later blog post I’ll discuss the Notch Signaling Pathway and Notches 1, 2, and 4. In another I’ll talk about some of the disease-causing variants the field is already familiar with. But I’ll finish today with a brief description of what Notch3 is meant to do.
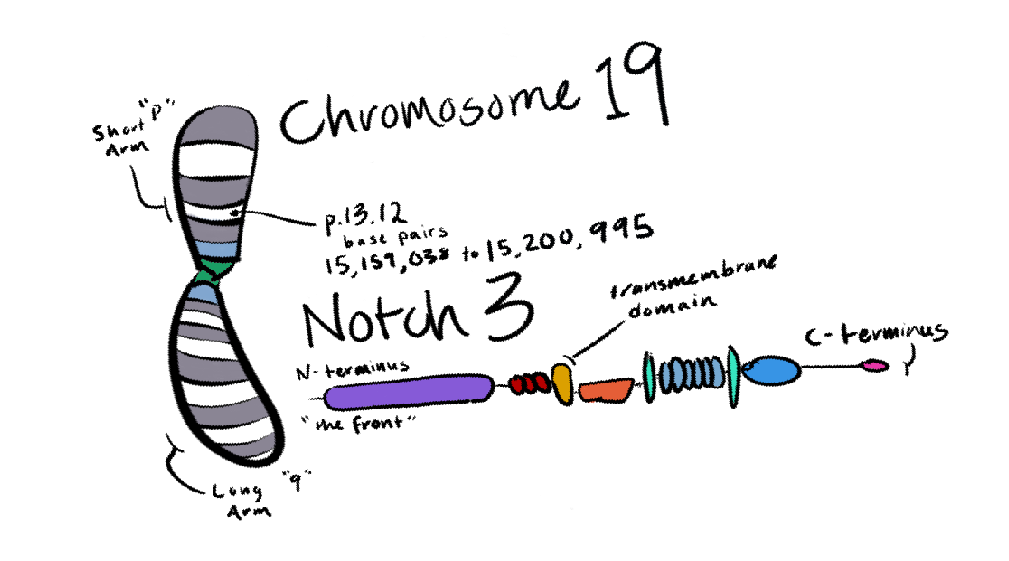
Notch3 lives on the membrane of vascular (blood vessel) cells, and it’s meant to receive a signal from a neighboring cell. Notch3 is then meant to cleave itself, with part of it migrating to the cell’s nucleus, contributing to the survival and normal function of the cell.
A Notch3 variant that results in CADASIL pathology either doesn’t cleave itself or does so incorrectly, resulting in accumulation of the protein between cells and the prevention of the signal pathway completing. In my next post, I’ll go over how and why this might happen and how the BBI and Starita Lab will address this problem!
Thanks for tuning in for an introduction to CADASIL and Notch3.
- Nahum Smith